Products are currently available as R&D samples only.
Product
R&D Samples
Description
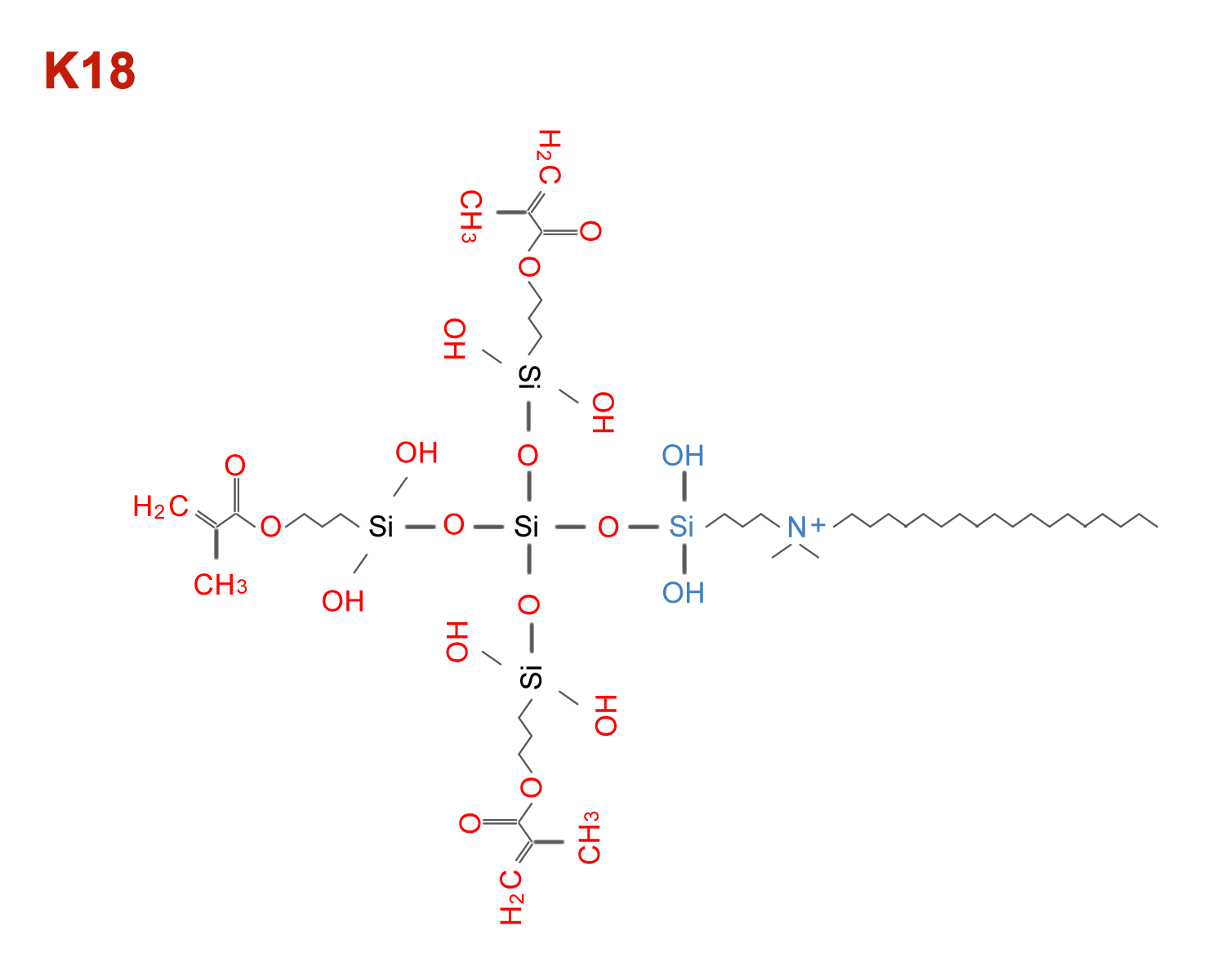
K18 QAMS
Available
K18 antimicrobial for use in adhesives, sealants, cements, endodontic sealer
K18 QAMS-M
Available
K18 MMA for antimicrobial orthodontic acrylics, dentures, oral devices, partials
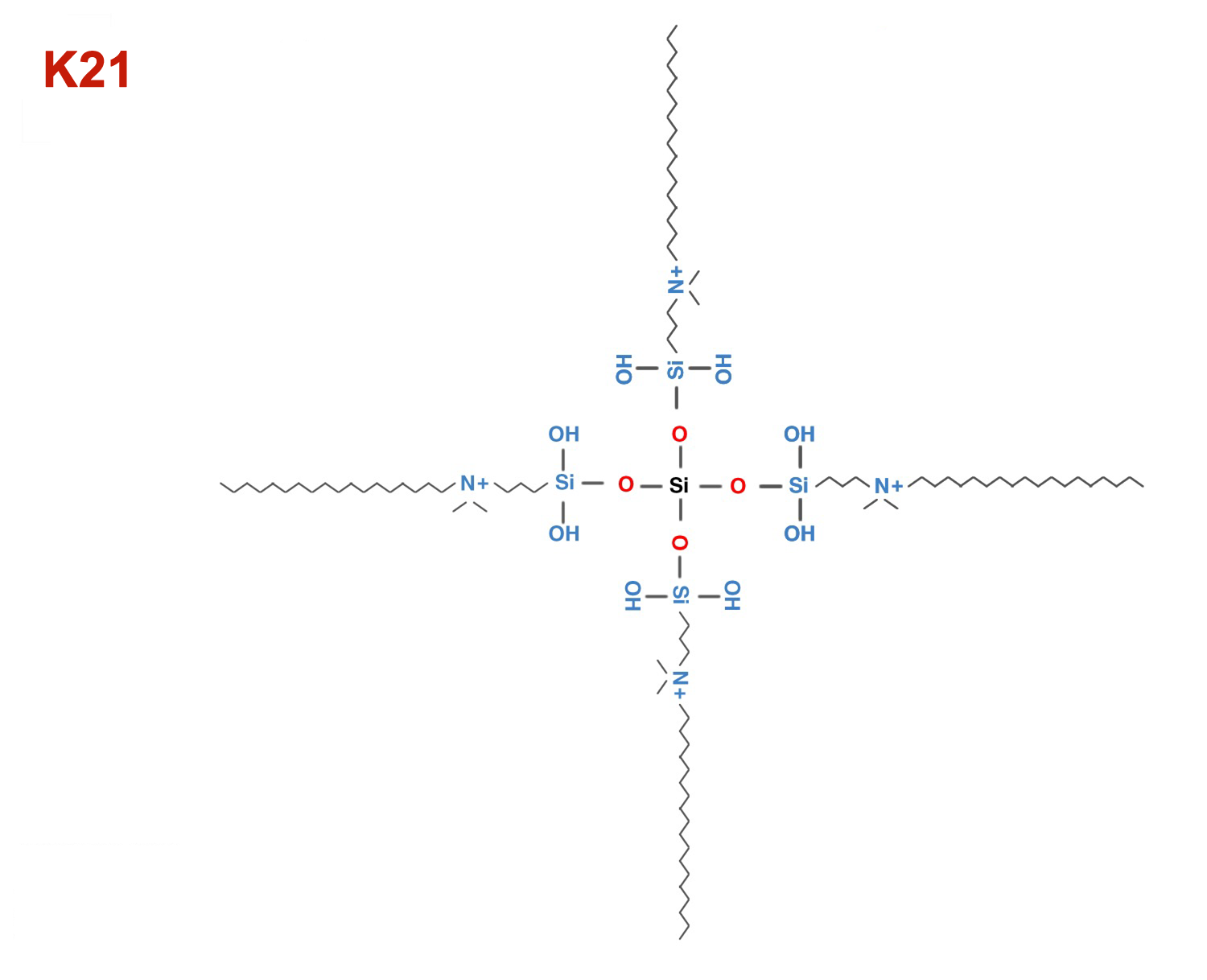
K21 QAS
Available
K21 antimicrobial for cavity cleanser, sealants, mouth rinses
K21 QAS D
Available
K21 antimicrobial
RD70
Available
RD70 antimicrobial silica with 17.5% K21 for silicone fluids for silicon mouth guards, resin systems, 15 micron
G30
Available
G30 antimicrobial dental glass or silica for composites and resin systems with RI 1.530 and 0.7 micron
G41
Available
G41 antimicrobial dental glass or silica for composites and resin systems with RI 1.541 and 0.7 micron
G50
Available
G50 antimicrobial dental glass or silica for composites and resin systems on AEROSIL OX 50 (Silica)
G55
Available
G55 antimicrobial dental glass or silica for composites and resin systems with RI 1.555 and 0.7 micron
K18
Upon Request
K18 antimicrobial for use in adhesives, sealants, cements, endodontic sealer
K18-M
Upon Request
K18 MMA for antimicrobial orthodontic acrylics, dentures, oral devices, partials
K18 IBOA
Upon Request
K18 IBOA antimicrobial isobornyl acrylate for use in 3D printing resin systems
1-7-1
Upon Request
S171 advanced antimicrobial nanoparticle silsesquioxane with K18.5 methacrylate for use in all resin systems 400-600nm
K18 QAMS molecule has been previously cleared as a component of a dental sealant (FiteBac CC OrthoSeal in K210115) and as a biofilm reducing component of dental resins (Lang Orthodontic Acrylic 2 in K163482).
PLATFORM CHEMISTRY
This video explains FiteBac’s antimicrobial technology and how it functions on a molecular level.
IN VITRO STUDY RESULTS
This GIF shows biofilm grown inside a root canal as part of an in vitro study.
The bacteria are stained red and as our antimicrobial K18 is introduced, the bacteria die off.
LEAD ANTIMICROBIAL MOLECULES
Medical Device Master File Certificate
K-18 QAMS and K-21 QAS
FiteBac can provide access to published research that proves the effectiveness of our antimicrobial agents. They are already present in two FDA-cleared devices which others can cite as predicate devices to speed up FDA-clearance navigation.
Applications
FiteBac’s patented antimicrobial molecules are present in multiple dental devices that are cleared by the FDA and they are available for use in various areas of dentistry, such as:
Adhesives
Sealants
Cements
Endodontic Sealants
Mouth Rinses
Bonding Agents
Dental Glass
Silicas
Resins
Crowns
Dentures
Implants
Silicone Mouth Guards
Composites
Night Guards
Retainers
Orthodontic Appliances
Resin-Modified Glass Ionomers