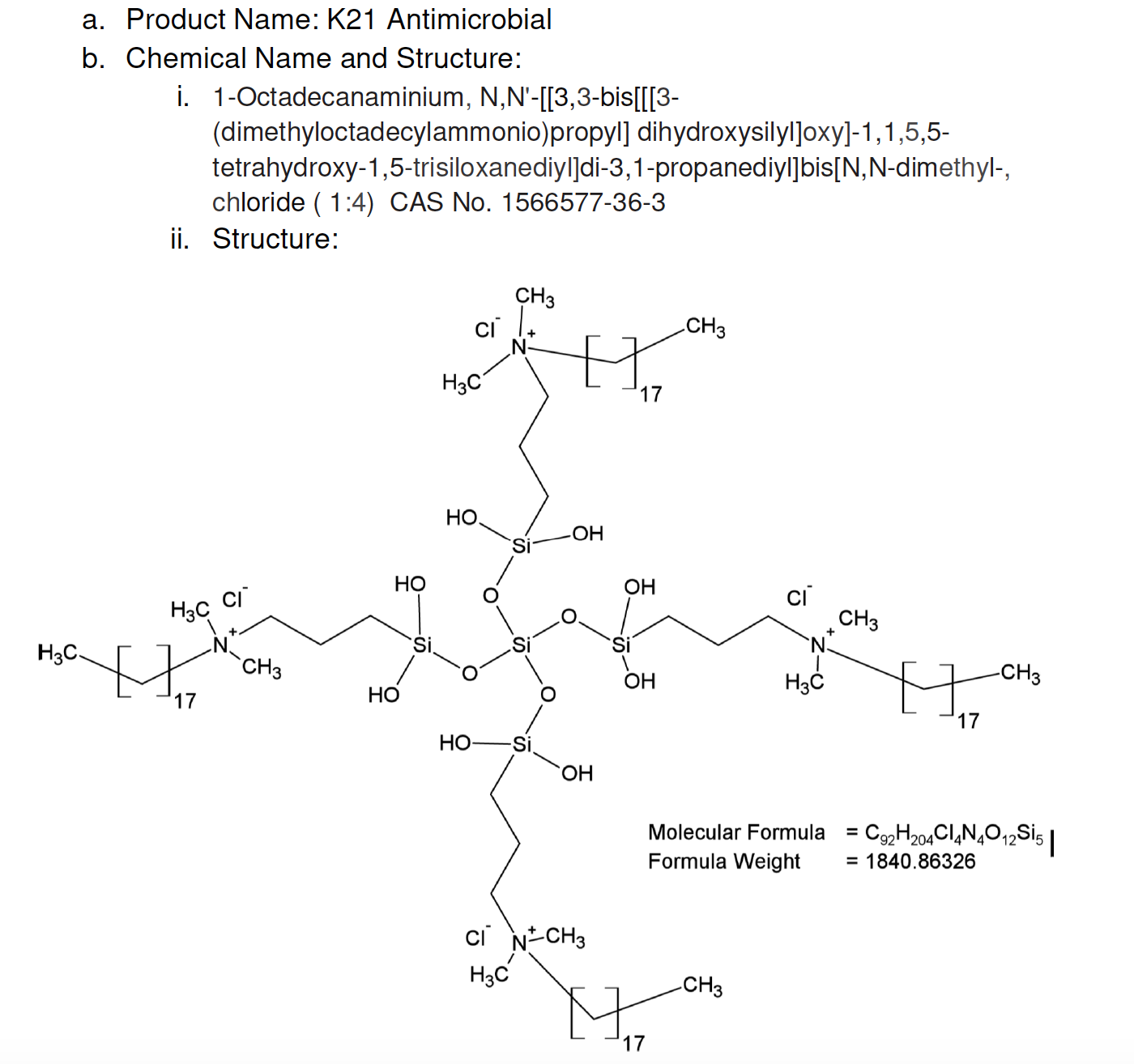
FiteBac Molecules K-21 & K-18
Dedicated to the research, development and commercialization of innovative healthcare products incorporating FiteBac® Technology, a superior approach to antimicrobial protection.
FiteBac® Lead Molecule K-21 presented to FDA February 18, 2016, in Pre-IND meeting. Application 128706.
K-21 for (P-IND) is NME that traverses the Medical Devices and Drug Application.

K21 recognized by FDA as a polymer with formula weight from 538.36186 to 1840.86326 FiteBac® Lead Molecule
K-18 (QAMS) has 510(k) Clearance for Dental Device.